Monday, June 29, 2009
HDLs and LDLs
Cholesterol and fatty acids are packaged into complexes known as lipoproteins with proteins by the liver in varying degrees of density, including chylomicrons (which are the least dense, largest, and contain the least protein) to LDLs (low density lipoproteins) which are slightly more dense than chylomicrons to HDLs (high density lipoproteins) which are the most dense and contain the highest levels of proteins. High levels of HDLs are good because the complexes carry proteins that aid in the absorption of cholesterol from cell membranes and transport back to the liver. Consequently, HDLs help lower blood cholesterol levels.
LDLs, on the other hand, function primarily to carry cholesterol to tissues. Excessive levels of cholesterol in cell membranes can be problematic because it increases the permeability of the membrane, possibly allowing unwanted, and perhaps harmful, materials to enter the cell. In addition, extremely high levels of LDLs in the bloodstream can promote a pathway through which LDLs are absorbed by the cell and oxidized, leading to the inflammatory response known as atherosclerosis. Conversely, HDLs can be potentially anti-inflammatory because they inhibit the oxidation of LDLs that leads to this atherosclerosis.
References: http://en.wikipedia.org/wiki/High_density_lipoprotein
The Candyman Can
Several groups of scientists are currently studying ways to prevent the blooming of chocolate. NMR analysis has been used to determine that certain additives slow the process. The chemical structures of the triglycerides in cocoa butter have also been studied using powder x-ray diffraction. Though the research is still young, the teams are looking for a way to produce chocolate containing cocoa butter that is already in Beta-6 form, thereby decreasing the likelihood of fat blooms. More information can be found at the websites listed below.
I will be leaving Siena in the morning, so this will serve as my last blog post. I have thoroughly enjoyed my time here at the UNISI. The people I have met and the experiences I have had will truly impact my outlook on life for years to come. Thanks to Daniela, Renzo, Gabriela, and all of our other professors from UNISI for making our time here as enjoyable as possible. Ciao ragazzi!
www.physorg.com/news1208.html
www.esrf.eu/UsersAndScience/Publications/Highlights/2004/SCM/SCM8
Cannabis Controversy
First of all, I am not condoning marijuana use in any way. There is a reason why it is illegal in many countries around the world and this is probably because of the psychoactive substance in the plant called THC. Tetrahydrocannabinol produces the high associated with cannabis and has been known to alter brain function (like short-term memory loss). However, a drug is something that can be abused and can alter body functions. One can classify coffee or many over the counter medicines as drugs but their legality is never disputed. There have never been any reported fatalities linked to the overdose of cannabis and one study reports that 1500 lbs of pot would have to be smoked in 15 minutes to die from its toxicity. Frankly, I think that is impossible (well, maybe not).
There is a strong argument for the tolerance of cannabis. The plant is used for many medicinal purposes like for the treatment of nausea, pain, and chronic illness. In fact, there has been some research performed that showed some extraordinary benefits for patients with cancer and AIDS who are given cannabis. THC also provided positive benefits for patients suffering from glaucoma, multiple sclerosis, Alzheimer’s disease, Tourette’s syndrome, Parkinson’s disease, ADHD, and depression. In fact, some studies were able to confirm that THC can even promote neurogenesis and neuroprotection (the production and protection of neurons in the brain). Another study confirmed that THC can prevent some types of oxidative damage from free radicals and may be even more effective than some antioxidants.
There are many issues and benefits of marijuana that I haven’t included in my brief blog. I just wanted to bring attention to the fact that we should not pass judgment on cannabis when its true effects are unknown. More research needs to be done to confirm the health effects of cannabis. Only then can we discuss whether or not it should be legal. Until then, I encourage people to go to Amsterdam and see the cannabis phenomenon for themselves. But be careful…if you try it you might suffer from some severe health benefits!
References: wikipedia.org
Gazing into the eye of a fly

In Dr. Baldari’s lab, they use a Scanning Electron Microscope (SEM) to analyze many of the biological specimens that they work with. The SEM is the next generation of microscopes because it uses a beam of electrons instead of light to form an image.
The SEM has allowed researchers to develop new areas of study in the medical and physical science fields.
It has many advantages over traditional light microscopes. It has a greater area of vision which allows the researcher to look at more of a specimen at a time. It allows higher resolutions and since the SEM uses electromagnets instead of lenses to focus the beam it gives the researcher greater degree of control in the magnification.
The SEM is an instrument that produces a large magnified image by using electrons instead of light to form an image. A beam of electrons is produced at the top of the microscope by an electron gun. The electron beam follows a vertical path through the microscope, which is held within a vacuum. The beam travels through electromagnetic fields and lenses, which focus the beam down toward the sample. Once the beam hits the sample, electrons and X-rays are ejected from the sample. Below is an image of the entire microscope.

Detectors collect these X-rays, backscattered electrons, and secondary electrons and convert them into a signal that is sent to a screen similar to a television screen. This produces the final image. Since the detectors are able to collect electrons from every direction, this allows the researcher to get a comprehensive 3-D image of the specimen.
Your Inevitable Doom
Arsenic has appeared throughout history beginning with the Arab alchemist Jabir who in the 8th century created a poison called arsenic trioxide. Arsenic trioxide is odorless, colorless, and leaves no traces in the body. On the other hand, the Joseon Dynasty in Korea used arsenic-sulfur to poison important political figures as a sort of capital punishment. Accused political figures had to drink a cocktail containing arsenic-sulfur called sayak. During Europe's Middle Ages, Elizabeth Bàthory (Hungarian countess) is believed to have used arsenic to poison her lovers. The poison was suppose to keep her lovers from leaving her. To be honest, I do not understand how killing her lovers is not the same concept as the lovers abandoning her. Furthermore, women in the Victorian era used a special powder to whiten their skin; the powder consisted of chalk, vinegar, and arsenic. The joke is that the powder only prevented aging through the absorption of arsenic into the bloodstream which then led to the user's death. In the art world, arsenic was present in an Emerald Green pigment used by impressionists. Rumor has it that Van Gogh's neurological symptoms could be due to his use of Emerald Green.
During the 19th century, the press provided circumstantial evidence on the possibility of mass poisoning in Europe. The large controversy over Arsenic poisoning had arisen from Karl Scheele's first synthesis of arsenic greens in 1778. This discovery led to the use of arsenic greens in wallpaper. By 1863, the United Kingdom produced 500-700 tonnes of arsenic green. German chemist Gmelin along with Italian chemist Gosi discovered that in damp environments, inorganic arsenic is transformed into a gas known as trimethylarsine. At this time, children were the number one victims of arsenic gas poisoning due to the green wallpaper used to decorate their rooms.
The lesson to learn here is that everything in life can lead to your death and/or recovery. All you need to do is sit back and relax. As one individual brilliantly said: “you have to die from something” (I do not remember the exact quote). Haha!
References
emedicine. Toxicity, Arsenic. http://emedicine.medscape.com/article/812953-overview (accessed
June 29, 2009).
Inventor Spot. Arsenic: Is This Ancient Poison a Modern Remedy? http://inventorspot.com/articles/
arsenic_ancient_poison_modern_remedy_24090 (accessed June 29, 2009).
Popular Science. Killer Wallpaper. http://www.popularscience.co.uk/features/feat17.htm (accessed
June 29, 2009).
Sunday, June 28, 2009
NMR analysis in depth
All of the analytical techniques have its limitations and its advantages. Liquid or solution-state NMR requires that the sample be soluble at some concentrations but when the analyte´s molecules weight passes the 30kDa the techniques becomes very complicated. There is a size limitation for this NMR technique. Solid NMR does not require the sample to be in solution, liquid state or any different structure, this allows for much more efficient and direct analysis. Solid NMR permits to analyze samples bigger than 100kD. Solid NMR, as every technique, also has disadvantages when compared to solution-state NMR. The main difference between the two techniques is the random motion, mobility and rotation of the sample in the liquid state. Solids lack these rotational and translational movements even though some molecules can still have some sort of rotation and movement, like the rotation of the methyl groups or the flip of the rings.
The advantage of solid NMR is that since the molecule are mainly fixed in the space, clear and defined lines appear in the NMR Spectrum. The disadvantage is that information regarding the orientation of the molecule in the space is not avaliable. This disadvantage somehow limits the analysis of biological macromolecules.
In Solid-state NMR anisotropic nucelar interactions (directionally dependent interactions) influence the behavior of the system, of the nuclear spins. There are two anisotropic interactions characteristic in solid NMR: chemical shift anisotropy and internuclear dipolar coupling.
Microscopes Should be Sold on eBay
TEM can be used to analyze a variety of sample including samples from medical and biological sciences. For example, TEM can be used to observe the morphological changes of human tissues when treated with drugs. Tissue samples have to be cut into extremely thin sections to be viewed by this microscope. The size requirement for TEM is actually an advantage for researchers who have difficulty obtaining samples in the first place.
References
Intertek Northwest Technology Centre. GLP Tissue Microscopy. http://www.intertek-cb.com/ nwtc/biotemlab.shtml (accessed June 28, 2009).
Nobel Prize in Physics. The Transmission Electron Microscope. http://nobelprize.org/educational_ games/physics/microscopes/tem/index.html (accessed June 28, 2009).
University of Nebraska. Transmission Electron Microscope (TEM). http://www.unl.edu/CMRAcfem /temoptic.htm (accessed June 28, 2009).
Red Red Wine You Make Me Feel So Fine
The process begins with sampling of the grapes. The experienced wine producers choose which vineyard to pick from at what time, based on what has worked best in the past. The rows of grapes that are picked are also chosen strategically, as well as which grapes in the bunch will be used. The grapes in the center of the bunch are generally most suitable for wine making. Once the grapes are collected, they are put on dry ice until they are put into the destemmer. The destemmer uses a vacuum to pull the grapes in and leave the stems. After they are destemmed, the white grapes go straight into the press, while the red grapes are put into tanks. Next, they are prepared for fermentation by adding potassium metabisulfate. This compound kills the natural yeast from the grapes so that the fermentation can be controlled. Once this has been removed, other forms of yeast such as Sacchoromyces cerevisiae are added to begin controlled fermentation. Afterwards, the grape skins are separated from the liquid, and oxygen is added to the tank to aid fermentation. Then the liquid is recombined with the grapes, and the combination is put into the press. The liquid is transferred to a new tank, and the wine that comes out first is the best product.
Not only did Natalie get to witness the production of the wine, but she also helped to analyze it. In the lab, the FOSS WineScan apparatus was used to analyze percent of alcohol, pH, acidity, Brix (the amount of sugar in the grapes), sulfates, and more. Another analysis technique that is conducted is testing for cork taint. When the corks arrive, measurements are taken and then a handful of them are put into full wine bottles. After 24 hours, the corks are removed and the researchers line up to smell for cork taint. Bad corks may have fungus that reacts with the wine to produce unpleasant smelling phenols. The bad corks may be disposed of so that they will not taint the wine. Numerous other analyses are done in the lab as well. Who knew that so much effort has to go into creating the most abundant beverage in Italy!
The Science of Boozing
There's 6 basic steps to brewing beer, and they are detailed below, courtesy of blogaboutbeer.com. The hard work that goes into making a beer is unbelievable, and the science behind it is fascinating.
The Chemistry of Making Beer (www.blogaboutbeer.com)
1. Malting: “Malting” is the controlled germination of barley (say what?). After steeping the barley in water, the grain is spread on a malting floor and allowed to grow until it is modified. Natural enzymes transform the endosperm from complex to simple starches. The grain is dried at high temperatures and milled.
2. Mashing: Bringing the “mash” of grains to between 148 and 158 degrees activates a pair of related enzymes that liquefy and reduce the now-soluble starches into maltose and other simple sugars.
3. Lautering: Once all reducible starches have been converted, the mash is heated again to 170 degrees. The liquid is usually drained out through a bed of the original grain; the husks are then rinsed (”lautered”) thoroughly with more hot water. The collective runoff from the mash is called “wort,” and it constitutes what will become the finished beer.
4. The boil: Achieving clear beer with a firm, foamy head is largely a function of removing most – but not quite all – proteins from the original mash. Proteins, when boiled, will coagulate and settle out of the liquid (forming a gummy mass called “trub”); this action is called the “hot break.” Boiling is also necessary to extract important flavoring agents, called alpha acids, from hops. For the most part, the longer the wort is boiled, the more efficiently a given amount of hops can bitter a quantity of beer. Boiling even longer can produce caramelization of sugars in the wort.
5. The cold break: As soon as the boil is complete, the wort is quickly cooled; this removes even more undesirable proteins and tannins out of the wort. This time the process is called the “cold break,” and the residue is called “cold trub.”
6. Pitching the yeast: Perhaps the most important key to making good beer is to keep wild yeast and bacteria from gaining a foothold in your brew before the preferred yeast does. This is done through good sanitation and proper “pitching” of a sufficient quantity of carefully cultivated beer yeast. When the wort is cooled, a thick broth of cultivated yeast is added.
- A. The lag phase: The yeast immediately begins to absorb oxygen. Enzymes facilitate yeast’s intake of glucose, more complex sugars and other nutrients. This happens in a few hours.
- B. The respiration and fermentation phases: With sufficient food reserves stored away, the yeast begins to reproduce by “budding.” It absorbs all the remaining oxygen in the wort and uses it and other nutrients to produce new “daughter” cells. Once all oxygen is absorbed, reproduction halts and fermentation proper begins. In a simplified explanation, yeast turns one molecule of glucose into two molecules each of ethyl alcohol and carbon dioxide.
- C. Clarifying and carbonation: Once all available fermentable sugars are consumed, fermentation grinds to a halt and the yeast begins to go dormant. The beer is clarified by storing in a cool, still, sterile environment. It is now nearly free of clouding agents and is clear. It is also flat. During the whole fermentation process, the huge amount of carbon dioxide produced has been allowed to escape through a gas vent, while the alcohol has been preserved in an otherwise closed environment. To achieve carbonation, brewers inject carbon dioxide to the desired level.
The NMR Spectrometer: A Brief Tutorial
First, the sample and an appropriate solvent are added to a tube made specifically for the NMR spectrometer. Then the tube is sealed and shaken. The tube is lowered into the machine at the probe head. Next, a superconductor generates the magnetic field. The magnetic field generated is quite strong (generally between 200 MHz and 900 MHz) and this is why we had to leave our credit cards in another room. Temperature control is a key issue for the superconductor, so the spectrometer has various components to monitor and control the temperature.
The next major component is the spectrometer cabinet. The role of the spectrometer cabinet is to provide three radiofrequency channels: the observe, the lock, and another channel for decoupling. These frequencies are controlled by the computer and are transmitted to the probe head (where the sample is located) and then after some amplification, they are transmitted back to the computer. The probe essentially delivers radiofrequency radiation to the sample and receives the signals from the sample.
Lastly, the computer collects the different frequencies and produces the data for us to analyze. Specifically, the computer has its own components to accumulate the NMR signal and process the NMR spectra. Also, these computers must have high computing speeds and high storage capabilities.
I have described the NMR spectrometer very briefly, but I recommend reading about the components and functions of the NMR in detail. It would be even better to see the actual machine and I am sure many of us will have the opportunity to see it in the future.
References:
media.wiley.com/product_data/excerpt/73/.../3527310673.pdf
http://www.varianinc.com/cgi-bin/nav?corp/businesses/nmr/components&cid=KOHOQNMIFIH
Radiocarbon dating
Developed by J. R. Arnold and W. F. Libby in 1949, radiocarbon dating relies on a simple natural phenomenon. The earth's atmosphere is struck by cosmic rays from space, producing carbon 14, an unstable isotope of carbon. Over time radiocarbon atoms decay into nitrogen atoms. This tendency to decay, called radioactivity, is what gives radiocarbon the name radiocarbon.
Radiocarbon dating works by measuring the ratio of radiocarbon to stable carbon in a sample. In the case of the Shroud, this is done by accelerator mass spectrometry. From this measurement, the age in radiocarbon years is calculated. The final step is calibration, after which an estimate of the age of the sample is determined.
There are imitations, however, to radiocarbon dating. Larger samples are better off since purification and distillation remove some matter. And although radiocarbon dating through TAMS is an option (as was used for the Shroud), it is very expensive and still somewhat experimental.
Sources:
http://id-archserve.ucsb.edu/anth3/courseware/Chronology/08_Radiocarbon_Dating.html
http://www.biblicalchronologist.org/answers/c14_method.php
Give the B-Ball its B-Bounce
Anyone who watched the game would tell you that I never dribble the ball. Never. I don’t have some kind of aversion to the action, it’s simply not in my muscle memory, since I never grew up playing the game (I grew up with Netball, similar concept, but no dribbling.). Watching the others do it so naturally brought a question to my mind; what gives the ball its bounce?
I do remember from physics and chemistry, both, the idea behind elastic collisions. In the scientific sense an elastic collision is one in which there is no loss of kinetic energy, there may be a transfer of energy from one object to another, but the resulting movement is reactionary. In that sense too, I remember that Newton teaches us that each reaction has an equal and opposite reaction. So I figure that along with the elastic properties of the ball part of the situation is as follows: the ball is driven down [forcefully] against the ground. The ground cannot recoil to absorb the impact, thus the ball bounces back against the equal and opposite force now given by the sturdy ground. I wasn’t completely convinced by my own simple answer, though, and decided to investigate a little further into the matter.
I found I had two ways to approach the matter, the first is to strictly consider basketballs, and the other was to consider bouncy-balls in general. Apparently the answer to one is not the answer to the other, so I will talk about the prior. I had suspected that the answer to the bounce lie in the chemistry of the rubber, I was wrong. Though some balls, no doubt, do have more spring because of their rubbery exterior, the secret to the bounce lies beneath the surface too, quite literally. The anatomy of the ball, then, can shed a little more light on the subject. The typical make-up of a basketball ball from exterior to interior is an outer covering, usually leather or rubber, which wraps over layers of fiber that in turn covers an inflatable “inner bladder.” (1) The air, in this case, lends the power to the bounce. When a ball strikes the court, the air inside the ball compresses and absorbs the energy of the strike. The ball recoils from the compressed airs need to return the ball to its original shape, and the air contained to its original energy. (2) This is also the reason that the ball does not bounce as well when it is not fully inflated. The same effect can also be given by cooling the ball. (2) This results in less thermal energy of the molecules of air inside the ball, and thus decreased pressure of the molecules hitting the inner surface of the bladder.
The outer surface is not without contribution, however. Were it not for the elastic properties of the outer surface, the ball would not be able to rebound and regain its shape. The air is only part of the equation. Trying to bounce a ball made of glass would obviously not have the same result. Having a brittle shell, despite being filled with air, would cause the ball to shatter.
So what are the lessons learned? Fill yourself with air and have a bouncy outer surface in order to jump back when life hits you hard… Take a deep breath, contemplate the situation before reacting. Don’t let the small things cut you deep; let them bounce off of you. Maybe then you will have more ups than downs in the basketball game that is your life.
(1) http://en.wikipedia.org/wiki/Basketball_(ball)
(2) http://www.howeverythingworks.org/bouncing_balls.html
Why are you so red?
Alcohol flush reaction is a condition in which the body cannot break down ethanol completely, due to the inheritance of mutated ALDH2 that allows a buildup of acetaldehyde in the blood and tissues after the consumption of alcohol. High blood acetaldehyde levels are believed to be the cause of these flushed symptoms. About half of the Asian race is considered to be sensitive to alcohol because of this condition.
Never fear, there is hope for those that want to control their Asian Glow. Taking low doses of heartburn medicine containing ranitidine or famotidine, such as Zantac or Pepcid AC, may help relieve symptoms if taken before drinking. Also alcohol-flushing tends to lower the consumption of alcohol within individuals, therefore, people that have this reaction are less likely to become alcoholics. Who knows? Asian Glow may have saved your life.
http://www.springerlink.com/content/f7lt18165773j81l/fulltext.pdf?page=1
http://en.wikipedia.org/wiki/Asian_glow
Dermatology of David: Poultice of Cellulose
In discussing the cleaning of the Statue of David in class Thursday, we learned that there are many successful techniques that can be applied. Conservationists can use an anionic exchange resin, solvent gel, white spirit, and…poultice of cellulose? What is poultice of cellulose? I imagined some kind of green jelly spread, while Dr. Norton imagined the soft stone tool a person uses to scrape dead skin off their feet. Courtney, the only person in the group with art conservation experience, did not know the answer either. Nobody had a clear idea of what poultice is, so I was assigned the task of discovering what exactly a poultice is.
There are multiple kinds of poultice. Cataplasm, another name for poultice, is a medicated spread put on cloth over a part of the body that is injured. I didn’t think David was in such pain. The second definition of poultice is a porous, solvent-filled solid used to clean stone- much better.
In class, we discussed how a stone becomes stained with the statue, The Thinker. Stone is a porous substance that becomes stained once a solution goes through the surface and evaporates to leave a solid solute behind the stone layer. Poultice is made from a malleable and porous material, usually paper, whiting, or flour. In our example, the porous material is a cellulose pulp. The sponge type solid is filled with a solvent (alcohol, ammonia, acetone). The poultice and solvent are applied, and the solvent dissolves through the stone pores and equilibrates between the stone and poultice boundary. Once the poultice is removed, so it’s a portion of the dissolved stone solute stain.
Besides cleaning marble or other stones, poultice of cellulose is also used to clean wall paintings and to conserve textiles. It turns out poultice is nothing like what we imagined, but it is an effective cleaning method nonetheless. Now we now that David will never need a dermatologist because his pores remain clean with poultice of cellulose.
References:
Grissom, C.A. “Methyl Cellulose Poultice Cleaning of a Large Marble Sculpture.” VIth International Congress on deterioration and conservation of stone. (1988), pp. 551-562.
Lemiski, Shawna. “An Investigation of Poultice in Materials for Textile Conservation.” Textile Conservation Newsletter. (1998), pp. 1-15.
Luigi Dei, Andreas Ahle, Piero Baglioni, Daniela Dini and Enzo Ferroni. “Green Degradation Products of Azurite in Wall Paintings: Identification and Conservation Treatment.” Studies in Conservation, Vol. 43, No. 2 (1998), pp. 80-88.
A Method of Food Preservation
To preserve the freshness of fish and other foods, it may be possible to use enzymatic manipulation of the fish to prevent or revert the degradation of its compounds. For example, the fish can be placed in a solution containing an inhibitor to the enzyme that creates hypoxanthine. Or, an enzyme can be added that reverses the formation of hypoxanthine. In this manner, fish can be preserved longer from the smell standpoint. This method obviously will not prevent any other forms of spoiling food, but can be employed to decrease the strength of the typical "fish" smell.
Fried Rust Could Save Millions
After reading the articles about Napoleon’s supposed arsenic poisoning, I was curious about the history of arsenic poisoning but was surprised to find out that this issue is a modern day problem. In fact, millions of people are drinking water contaminated with arsenic in Bangladesh, Cambodia, India, Myanmar, and Vietnam. Arsenic found in sediment is anaerobically metabolized by bacteria and dissolved into essential water sources. This event has been known to be the biggest mass poisoning in recent history.
In order to find solutions to this serious problem, researchers had to think both scientifically and economically. Many of these rural areas lack the resources needed to support a clean up process. A research team at Rice then attempted to use Rust nanoparticles to extract the harmful arsenic. An iron oxide known as magnetite was used for its obvious magnetic properties as well as the nanoparticle ability to exert forces on themselves. Arsenic binds strongly to iron oxide. Once bound, these particles simply require a magnet to extract from the water. After a few nanoparticles are pulled by the magnet, the nanoparticles pull on each other to finish the job. “Small particles are great- high surface area, high contact, short diffusional times between particles to suck up things…” says Paul Laibinis, an engineer from Vanderbilt University. Best of all, these iron nanoparticles can be obtained from heating rust in olive oil. This method can be used to purify 50 to 100 L batches of water. Personally, I love this combination of modern science with applicability that can really help millions harmfully affected by arsenic.
(http://209.85.129.132/search?q=cache:G9gpRlpHDoAJ:www.reactivereports.com/chemistry-blog/fried-rust-could-prevent-arsenic-poisoning.html+arsenic+poisoning+chemistry&cd=1&hl=en&ct=clnk&client=safari, http://www.physorg.com/news157133188.html, http://www.scientificamerican.com/article.cfm?id=rust-could-be-the-key-to)
Saturday, June 27, 2009
Capillary Electrophoresis
The main component of the apparatus is a silicon capillary with a bore diameter ranging from 50 to 100 micrometers. Since the surface area to volume ration is so high, the capillary can dissipate heat more easily than more standard gel electrophoresis. This means the apparatus can be run with much higher electric potential difference or voltage. Gel electrophoresis is run around 6 volts, while capillary electrophoresis can be run between 5 and 30 kilovolts. The interior of this capillary is made up of silanol (SiOH) groups that, when deprotonated, form a negatively charged tunnel. At this point an electrolyte buffer in pumped into the capillary. The positive ions in the buffer form a layer of charge over the negative silanate. When the voltage is applied across the length of the capillary, these positive ions migrate from the anode to the cathode and carry the whole solution along. This migration is called the electroosmotic flow (EOF), and is dependant on the strength of the voltage, the buffer used, and the quality of the silicon capillary. When a very small amount of the sample is added to the capillary, it is forced to move with the (EOF). The speed that components travel is dependant on their charge to mass ration. Very small very positive components travel the fastest, and will be detected first. Neutral components travel around the speed of the EOF depending on their mass. Negative components are retained in the capillary the longest due to their attraction to the anode. At the outlet end of the apparatus is a small length of exposed capillary, where UV-vis or other forms of detection can be done.
Capillary electrophoresis (CE) is similar to HPLC is its qualitative determination of components. One advantage to CE is that very little carrier solvent used. Also, in CE the detection method is attached to the separation method, unlike HPLC were the detection is separate. This allows for clearer differentiation of the sample components. The disadvantage is that quantitative analysis is not accurate with CE. Part of this comes from limitations of UV-vis detectors for cells shorter that 1 cm; 50 μm in the case of CE. Also, since the components are not traveling at the same as in HPLC, the integrations of the peaks are not very helpful. Another limitation is that CE cannot be used for physical separation. The sample is just too small to make separation meaningful.
http://en.wikipedia.org/wiki/Capillary_electrophoresis
Friday, June 26, 2009
Bacteria and Art: Enemies or Allies?
When you walk through a grocery store, you will undoubtedly come across the aisle with cleaning products plagued with “Anti-bacterial: Kill 99% of germs” across their labels. And that’s because all bacteria is bad, right? Well, not necessarily. Earlier this week we discussed the use of bacteria in a positive light- restoring and preserving art, and thus cultural heritage.
We spoke of a particular case in which bacteria, specifically a strain of Pseudomonas stutzeri, were used to destroy the impenetrable cross-links between a piece of cloth and glue that had entrapped a fresco. But what was perhaps more interesting to me, was the side-mentioned story of bacteria in restoring statues.
As we have traveled Italy, we have seen our fair share of statues and fountains all of which are subject to the unforgiving conditions of Mother Nature. When acid rain, or sulfuric acid, strikes the statue a reaction occurs transforming the calcium carbonate (insoluble) of the statue into calcium sulfate (soluble). This is a problem for two reasons: calcium from the statue is removed and unsightly black ‘crust’ is formed. Scientists have turned to two different bacterial strains to solve the problem, Bacillus cereus and Desulfovibrio vulgaris.
Calcium sulfate can crystallize and during this process airborne pollutants can be enclosed in the matrix. This is essentially what comprises the black ‘crust.’ Desulfovibrio vulgaris is a sulfate-reducing bacterium that is used to digest the calcium sulfate and remove the discoloring of the statues. On the other hand, Bacillus cereus is used to restore the calcium loss. These bacteria, which naturally produce calcium accumulations, are allowed to grow on the surface of the statue. Once the food supply runs out, the bacteria die and are removed, leaving behind a coating of calcium protecting the statue from further deterioration. These two strains work hand-in-hand; while one restores, the other preserves.
The case study just offers more proof of the degree to which art and science are intertwined and helps to challenge the “bad reputation” bacteria is known for.
For further reading: Jose Luis Ramirez, Maria A. Santana, Ivan Galindo-Castro, Alvaro Gonzalez, The role of biotechnology in art preservation, Trends in Biotechnology, Volume 23, Issue 12, December 2005, Pages 584-588 http://www.sciencedirect.com/science/article/B6TCW-4HCMS4Y-1/2/74a1aa67e35c05d6fe3b15d9089fa2e4
X-Ray Diffraction Satisfaction
Next, we moved to a room that housed the lab’s two polarized-light microscopes. Though all of us had used microscopes in the past, it was an entirely new experience to work with such nice equipment. Polarized-light microscopes allow scientists to observe both transmitted and reflected light, thereby allowing for microstructural identification. After looking a few generic samples of mineral and getting a feel for the instrument, Dr. Mugnaini introduced us to her most current cross-sectional analysis: pigment layers from 13th century frescoes that were recently discovered under Siena’s Cathedral. We looked at samples of flesh, sky, and gold leaf from the frescoes. As we talked, it became increasingly apparent that Mugnaini was completely enthralled with her work; her enthusiasm was contagious! She pulled out samples from Ghiberti's Gates of Paradise and Donatello's David for us to observe and, if we would have had more time, she would have spoken to us all afternoon. It was my favorite academic experience of the trip thus far and made me excited about the prospect of working with people like her in the future.
Thursday, June 25, 2009
Color Blindness is Great!
Bragg’s Equation:
2 d sinø = λ, where:
d = diffraction gradient
ø = angle of beam
λ = wavelength
The outcome of our pigment, which was a red color, was mercury sulfide or vermillion....ironic, huh?
Wednesday, June 24, 2009
Fresco Painting
There are two types of frescoes. In buon fresco the pigment in applied directly to the wet plaster. As the plaster dries the pigment is incorporated into the wall making it both stable and sensitive to environmental conditions. In secco fresco, the pigment is applied to dry plaster so a binder such as egg white is needed. The plaster used is a combination of slaked lime and sand. Slaked lime is a paste or putty made of 1 part lime (CaO) to 2 or 3 parts water. It is a suspension of calcium hydroxide crystals (Ca(OH)2), and in order for it to be of the correct consistence it has to be produced over a period of at least 6 months if not longer. The first few layers of plaster are a 1 to 2 ratio of plaster to sand and are allowed to dry without pigment. A drawing is made on the last dry layer, and a paper guide is made from it. The most important layer of plaster has significantly less sand that the others, and is only applied in small areas at a time. For buon fresco, the pigment has to be applied within the first hour before the plaster dries. As the plaster dries the calcium hydroxide reactes with carbon dioxide to form calcium carbonate.
Ca(OH)2(s) + CO2(g)→ CaCO3(s) + H2O(l)
There were a limited number of pigments that maintain color in the basic conditions of the wet plaster, so certain colors, particularly blues, have to be added a secco.
http://www.mega.it/eng/egui/monu/buq.htm
http://www.wellesley.edu/Chemistry/Chem&Art/Topics/Pigments_Painting/Painting_pdf_material/fresco_lab02.pdf
http://en.wikipedia.org/wiki/Fresco
Tuesday, June 23, 2009
A Shock to the System
The first part of the experiment involves electrochemical stripping. This procedure can analyze many elements at a time, detect very minute amounts of metals, and is inexpensive to perform. They first start by taking 4 mL of wine and diluting it with 6 mL of a buffer which helps stabilize the pH of the solution. They then insert three electrodes into the solution: an auxillary, a working, and a reference. Next, they use a computer to set the amount and rate of current that will pass through the wine. After that, nitrogen gas is added while the solution is stirred to aid in the chemical reaction. Finally, the electricity runs through the system and the computer records the results.
During this reaction, the analyte (copper) is deposited onto the surface of the working electrode which has a strip of mercury inside. Later, this analyte is "stripped" off the electrode and the signal received determines the amount of copper present in the solution. After these two steps, the graph will ideally look like the head of a duck such as the one pictured below. The graph we saw did not have this shape however because wine is made up of many different substances, not just copper.

Overall, this experiment was really interesting and informational. I did not know very much about electrochemistry, but I came out of this lab knowing a great deal. Plus, Dr. Corsini geared this experiment toward what we were learning which was very helpful. I hope this lab will spark your interest too.
Monday, June 22, 2009
Wine: Alcohol, Antioxidants.....Metals?
One way to measure metal in wine is to examine the target hazard quotient (THQ) content in various wines. The scale is easy to understand. Any substance with a THQ over 1 is judged to be unsuitable for daily consumption. According to a study done by Dr. Declan Naughton and Andrea Petroczi, the high THQ levels if many wines (above 50 in many wines that were tested) can be mainly attributed to vanadium, copper, and manganese. Other metal ions in wine that also have high THQ levels are zinc, nickel, and lead. The source of these metals is relatively unknown. Some suggest the soil and others insist it’s the grapes themselves. Regardless, the high content of heavy metals in some wines is of major concern and it definitely strikes a blow into the ‘one glass a day is good for you’ theory.
Unsurprisingly, the same study by these two scientists shows that Italian wines have safe levels of THQ’s. Italy is the only European country to have this honor. On the other hand, it was concluded that other wine producing nations like France, Germany, and Spain (all of whom pride themselves on having the best wine in the world) have high levels of THQ. The results of the study by Naughton et al. are truly shocking. Manganese, for example, has been linked with Parkinson’s disease. Let’s just say I am happy that I am drinking Italian wines like Chianti Classico. However, next time, I will be wary when someone offers me a glass of Merlot.
http://www.webmd.com/food-recipes/food-poisoning/news/20081029/heavy-metals-found-in-wine
Dr. Shadi Shams’s lab.
Last week I had the opportunity to visit the labs of Dr. Tatiana Baldari. It was very inspiring to see lab techniques that I had learned about in freshman biology be used to work on scientific breakthroughs. The main techniques that we saw were the Western Blot followed by immunoblotting, which is the technique used to label the proteins that have been separated on the gel and membrane. Since Shadi has covered the process of Western Blotting and transferring the data onto a membrane, I’m going to look at the process which tags and identifies the proteins that are present.
Once a gel has been run and all the proteins have separated, the researcher needs to identify which proteins are actually present in that particular sample. The molecule used to tag the proteins is called an antibody. Antibodies are generated when a host species or immune cell culture is exposed to the protein of interest. Normally, this is part of an immune response, but in this case they are harvested and used as sensitive and specific detection tools. After blocking, a process that that prevents the antibodies from binding to the membrane directly, a dilute solution of the primary antibody is incubated with the membrane. The solution is composed of buffered saline solution with a small amount of detergent. This is sealed together over-night.
The following step is to use a secondary antibody to bind and tag the primary antibody so it can be detected. The membrane is first rinsed to remove any unbound primary antibody and it is then exposed to a different antibody which is directed at a species-specific portion of the primary antibody. Antibodies tend to come from animal sources, for example an anti-rat secondary will bind to just about any rat primary antibody. This allows some cost savings by allowing an entire lab to share a single source of mass-produced antibody, and provides far more consistent results. The secondary antibody is usually linked to biotin or to a reporter enzyme such as alkaline phosphatase or horseradish peroxidase. This means that several secondary antibodies will bind to one primary antibody and enhance the signal.
This secondary antibody is then detected using multiple methods. The most popular are radioactive and fluorescent detections. Other methods include a colormetric and chemiluminescent detection.
It was very interesting to visit Dr. Baldari’s (or should I say Dr. Shams’ due to her deep knowledge of how the lab worked) lab and see the different projects that they are working on. The main focus of the lab was the P66 Shc gene and different parts of the lab were working on different aspects of the gene. One was looking at how it affects cells in leukemia patients while another part looked at how they affected mast cells. I look forward to the chance of doing performing some of these techniques for myself tomorrow.
X-Ray Studies and the Health Dangers of Radiation
This Monday in class, we discussed the various forms of x-rays. In an x-ray, a stream of electromagnetic radiation passes through a collimeter. A colimeter is a group of metal plates with a channel through their centers. As the radiation passes through the collimeter, the plates absorb the stray electrons. The beam then goes through a filter, which typically is a metal that has an atomic number one higher than the element the scientist is trying to study. The stream of electrons collides with the atoms and displaces electrons surrounding them. The beam then either diffracts, absorbs or reflects to a different detection source depending on what kind of x-ray is being performed.
Specifically, Dr. Norton focused on x-ray fluorescence (XRF). In XRF, a detector measures the energy emitted and the electron transmission from one orbital to the next. In wavelength dispersive (WD) XRF, the electrons pass from the source, to the specimen, off of a crystal and to the detector, which measures energy based on the reflection angles. In energy dispersive (ED) XRF, the beam travels from the source to the object, to a silicon lithium detector and ends at the multi-channel analyzer. WD specializes in studying 1 element of interest while ED can locate and identify multiple elements at one time.
In class, we spoke about the dangers of x-ray radiation on the human body. Sameer said that it would take 10,000 x-rays in a period of 3 days in order to seriously endanger someone. Dr. Norton acknowledged that she did not know the details of x-ray exposure but that too much radiation is considered unhealthy. Being an x-ray technician, I was naturally interested in the specific health risks involved, and I performed some research to find out. According to the Helmholtz Association, a German research group, it would take 300,000 x-rays in one day to kill body cells. However, there are other rare risks involved with less radiation. While the cells’ atoms and molecules absorb the energy from x-rays, they can sometimes lose electrons, becoming ionized, and will break apart molecules within the body. The physical effects of deterministic radiation damage include nausea, cataracts, skin damage, and on occasion, death. Also, with stochastic radiation damage, cells can mutate and the number of mutated cells exponentially grows to cancer.
Medical and scientific staff use the lead apron in order to protect themselves from the dangers of x-rays. The jackets are made of lead because its high density and high atomic number effectively block most kinds of radiation from passing through. Even though the odds are in my favor and the major health threats are few and far between, let’s just say I will always be wearing my lead jacket when I am taking x-rays at work.
Beating a Dead Swordfish
Now back to Cinque Terre. We went to several restaurants, and due to their coastal location, most of us intended to try the seafood. Swordfish was on many of the menus and coincidently, ended up on several of our plates. There have been several discussions on whether or not mercury is going to kill us, but nothing has been said about why large fish have so much mercury despite a lack of HFCS and how mercury goes about wreaking havoc.
Large fish that are high up on the food chain have high levels of mercury due to something called biomagnification. Mercury, and methylmercury (the main source of mercury in fish) are fat soluble, and build up in the organs and muscles of smaller creatures, but organisms are not able to get rid of the mercury. As bigger fish eat smaller fish, they end up taking all of the smaller fish’s mercury, so a large carnivorous fish such as swordfish end up with the most mercury, which then gets passed on to the people who eat the swordfish.
When methylmercury is ingested, it is easily absorbed, and then binds to cysteine present in the body. From there, enzymes in the body confuse the mercury-amino acid complex with methionine, and it is readily transported throughout the body and across the placenta in the case of pregnant women.
http://en.wikipedia.org/wiki/Methylmercury
http://en.wikipedia.org/wiki/Bioaccumulation
http://www.healthobservatory.org/library.cfm?refid=102205
Sunday, June 21, 2009
Should You Choose Booze?
This week in my trip to the beautiful Dolomites, I was surprised to find myself in a very German town called Castelrotto. I knew geographically I was still in Italy, but it seemed that culturally, I was not. One difference I noted was the stronger inclination of the townspeople to drink beer rather than wine. We have thoroughly covered the winemaking process in class: from vine to bottle. However I thought it would be interesting to outline the process that is taken in the brewing of beer as well.
Though both drinks are made by fermentation from yeast, beer requires a more manipulated route of production usually using water, hops, and barley. After soaking the barley in water, natural enzymes start breaking down its complex starch into simple starches where it is then dried and milled. This process is known as malting. The mixture is then heated once again to activate another pair of enzymes that reduce the starches into maltose and even simpler sugars. This “mash” mixture is drained, leaving the original grains behind. After boiling once more, proteins masses are collected and removed. The boiling also aids the specific flavoring of the beer by releasing agents like alpha acids from the hops. Once cooled down, even more proteins and tannins are removed. It is important to control the wild yeast and bacteria that accumulate in the beer solutions by creating favorable environments for the desired yeast ferment the sugars. The carbon dioxide produced in fermentation is actually released, leaving behind flat beer. Manufacturers decided the amount of desired carbonation and inject it into the liquid. Though wine has a classier reputation, both beer and wine take intricate chemistry to develop their respective flavors, so I will leave the decision to you. Which is your favorite?
Fun in the Sun
Due to my personal skin tone, I usually do not apply sunscreen on the beach. I rarely sunburn and usually tan well. This time, however, I did get sunburned along with darkening my skin tone by many shades, almost to the tone of Helen after a glass of wine. All of my exposed body is much tanner, but my shoulders are a bit sunburned, so hiking back with a backpack was a bit painful, not mentioning the inability to take a hot shower.
Sunburn takes place when the exposure of UV light to skin is greater than the protective capacity of the individual’s melanin. In general, melanin content is greater in darker skinned individuals. This explains why I seem to sunburn less easily, though in this case due to a lack of sunscreen, I got sunburned anyway. Sunburn is triggered by direct DNA damage from absorbing UV-B photons. UVB light causes thymine base pairs adjacent to each other to bond together to form thymine dimers, which is a disruption in the strand, which reproductive enzymes cannot copy. This causes sunburn.
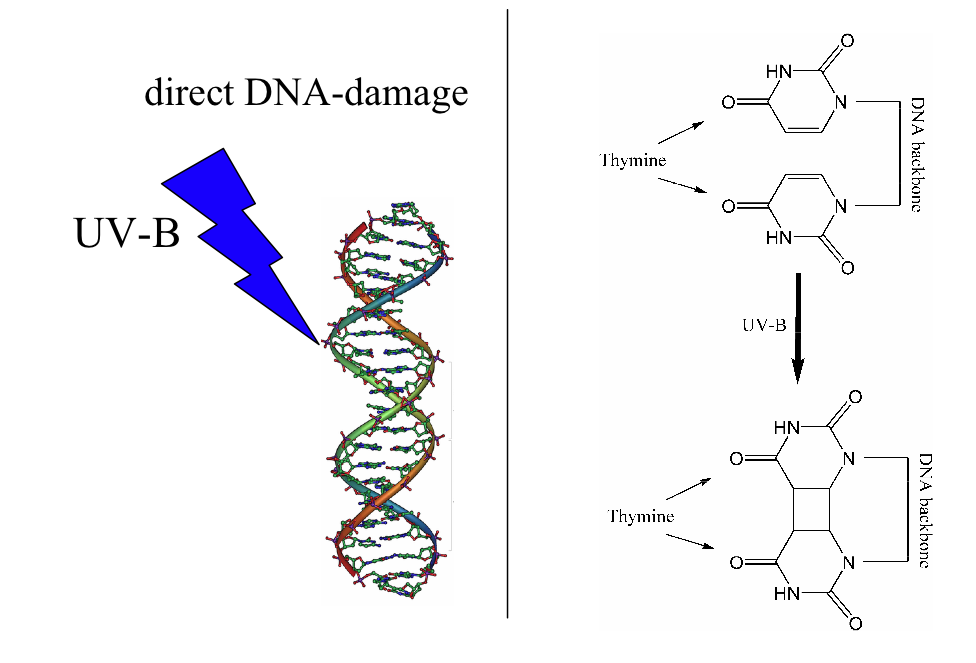
Works Cited
Parrish, John, Kurt Jaenicke, and Rox Anderson. "ERYTHEMA AND MELANOGENESIS ACTION SPECTRA OF NORMAL HUMAN SKIN *." Photochemistry and Photobiology 36.2 (1982): 187-191. Wiley Interscience. 23 June 2009.
Frozen Fritz
In order to get to Castelrotto, we took a train to Bolzano. In Bolzano, the South Tyrolean Archaeological Museum is the current home of the oldest human mummy in the world. His name is Ötzi, aka Frozen Fritz, and he is 5,300 years old.

He was found frozen in the snow on top of a glacier along the border between Austrian and Italian Alps. He was also found with various belongings, including a bow and arrows, and still wearing sheepskin leggings and a grass cape. Various scientific techniques were used on Ötzi, including radiocarbon dating to find his age, x-rays and CT scans to study his bones and what was left of his internal organs, DNA analyses to determine what foods were left in his stomach and intestine and if his DNA relates to any humans today (sadly, he has no living descendants), and finally, mass spectrometry analysis of his clothing to figure out what Ötzi did for a living.
Turns out, Ötzi was most likely a herdsman. Samples from his coat, leggings, and shoes were taken to study their proteins using Matrix-assisted laser desorption/ionization time-of-flight, or MALDI-TOF mass spectrom It involves a matrix made of three kinds of acid (in order to provide protons for the ionization of the protein sample), each of which has a high UV absorption rate (in order to absorb the laser irradiation effectively), low molecular weight (good for vaporization) and polar functional groups (so they can be used in aqueous solutions). The matrix is mixed with a protein sample, then allowed to dry and recrystallize on a metal plate. Then, a laser is shot at the crystals, the sample is ionized, and the molecules are accelerated into an electric field in a “flight tube,” where molecules are separated based on their mass/charge ratio. The differences in this ratio causes differences in flight times of the molecules. The mass spectrometry results revealed that the animal skins Ötzi wore were from the specific species of animals that were herded seasonally throughout the region where he was found. MALDI-TOF is currently used to check the purity of animal skin and fur products, such as cashmere.
What's the best part about Ötzi's discovery, you might ask? You can buy Iceman key chains, backpacks, cigarette lighters... and, there is even an Ötzi drink!

For more info...
http://www.microbiology.science.ru.nl/tech/malditof/
http://www.mummytombs.com/otzi/news.htm
http://www.bolzano.net/english/iceman.html
The Donatello Pulpit
Donatello's innovative work of art includes seven carved marble panels of the open-air pulpit that is located in the Duomo of Prato, completed in 1438. However, the pulpit had suffered from much deterioration since, due to its location outdoors. The central panel was in the worst condition as it was the most highly exposed to atmospheric agents, probably nitrogen oxides.
Because of the deterioration, many different methods were tried in hopes to preserve the artwork from even more degradation. After having no luck and additional degradation, the panels were removed and replaced by fakes.
Many think that the reason for further deterioration is due to the mixture of residues and degradation products formed which have led to deep cracks appearing all over the artwork. More specifically, architect Sanpaolesi's use of ZnSiF6 and MgSiF6 as a treatment for the deterioration had led scientists to hypothesize that these fluorosilicate compounds were the primary reason for the formation of the deep cracks. After performing the surface analytical technique, X-ray photoelectron spectroscopy (XPS), it was found that Sanpaolesi's treatment cannot be blamed solely for the formation of the cracks.
Procedures carried out to restore artwork should be properly tested prior to experimenting in order to avoid further deterioration. It just goes to show that one's best intentions can generate the worst results, a case shown in the restoration of Donatello's pulpit.
Here is a really cool diagram of how XPS works:
http://www.nuance.northwestern.edu/keckii/xps1.asp
Saturday, June 20, 2009
Rules inside the museums
The energy of the light can interact with the chemicals present on the paintings by exciting the outer electrons and therefore changing the conformation of the molecules by causing radicalic reactions whitin the surface of the paintings and making it so britltle that it cracks at the end. A big consequence of this is the fading of the pigments and dyes when exposed to the ultraviolet light. The light speeds the cracking caused by the addition of varnishes. The energy of light causes the bonds between the monomers of the cellulose constituting the paper to break, this is what causes the cracking. To prevent this kind of damage the museums reinforced their windows with a plastic material that absorbs UV light.
Another thing that is prohibited in the museums is to smoke, and again there is a scientific explanation for this besides the respect that you owe to other visitors that do not enjoy the smoke as much as you do. Dust and sand particles can damage the painting by altering the tone of the colors. Tobacco particles have the same effect and they are even harder to remove especially if the painting is not varnished.
Friday, June 19, 2009
Inorganic Biochem - Drug Delivery (Part 2)
Later, after Gabriella's lecture, I continued thinking about the concept at hand, and wonderd if it would be possible to build upon the idea of metal complexes for drug delivery in the following manner:
Doctors can inject a relatively large amount of a metal complex with inactive drugs into a specific location in the body, then can trigger the release of the drugs by a noninvasive delivery of a small dose of a non-metallic compound that will undergo ligand exchange with the metal complex, thereby releasing a small amount of the drugs. The parameters needed are that the ligands on the metal complex must be strong than water, yet weaker than the triggering compound. The metal complex will deliver the drug to the specific surounding area of where it was injected, and this injection need only be performed rarely, whereas the triggering compound can be taken orally as needed. This method can be used to provide frequent drug delivery in a highly specific manner (physical specificity) while minimizing the number of dangerous, invasive injections needed (into the brain, for example).